BETWEEN A ROCK
AND A REGULATORY PLACEBETWEEN A ROCK AND A REGULATORY PLACE
Changing the perception of RPs
from Revenue Preventers
to Revenue Providers
A Responsible Person’s “periodic assessment and documentation
of the effectiveness of learning”
May 2021




Madeleine Ault
Director and Senior Consultant
Being the Responsible Person in the Pharmaceuticals industry is often a thankless task. Until an inspection, of course, when deaf ears miraculously hear again… for a few weeks anyway.
In my time as an MHRA Inspector and now as a Consultant and Trainer, I see that dilemma frequently. The RPs are almost universally conscientious and motivated and do their best to drive real quality improvements in their business. But they often find that the business priorities tend to give lower priority and budget to Compliance than revenue-making activities. RPs consequently find it hard to maintain minimum regulatory standards, let alone be the catalyst for a high-quality culture.
This article will look at one particularly taxing aspect of an RP’s role, the periodic assessment and documentation of the effectiveness of learning. To the rest of the business, this one area can appear to be esoteric to say the least; but encapsulated within the regulations is a massive opportunity for the business to fully embrace a culture of quality improvement that extends far beyond pure compliance… and allows the RP to argue that they are far more than just a “Revenue Preventer”.
The Regulations
Demonstrating learning is required in GMP and GDP – ‘the effectiveness of training should be periodically assessed and documented’ GDP 2.4. The RP is responsible for “ensuring that initial and continuous training programmes are implemented and maintained” and an MHRA Inspector will expect to see this. In practice this means the RPs need oversight of all training and assessment that impacts on the quality of medicines. They will need to demonstrate this to the licence holder and the inspector.
The Cost of Poor Quality
The MHRA blogs always raise concerns from an inspector’s perspective. ‘It pays to be compliant’ by Michelle Yeomans, talks about the increased inspection fees for a company that is non-compliant. Bob Hayes of SeerPharma UK will be presenting at Making Pharmaceuticals in October on this very subject but with more of a focus on the very real costs to an organisation of poor quality. So, there is a lot more to learn.
https://mhrainspectorate.blog.gov.uk/2019/01/11/it-pays-to-be-compliant
https://www.makingpharma.com/speaker/bob-hayes/
The Opportunity
As a former MHRA Inspector and now dealing with a wide range of Pharma businesses and professionals through our consultancy and training, I get a unique insight into how certain behaviours and cultures translate into business effectiveness. It seems clear to me that those companies that embrace a culture of excellence with a true emphasis on education are opening-up opportunities unrestrained by the desperate need to meet the minimum levels of compliance needed by the regulator. Maybe you are doing a great job training your staff but want to do an even better job and see some real benefits within the business.
Excellence in Learning
We all know that learning is not just reciting facts. Getting your staff to pass a training assessment doesn’t guarantee they will carry that learning over into their role. Will they continue to carry out the procedure when no one is looking? Excellence in learning means you have staff who understand the ‘why’ as well as the ‘what’, and ‘how’ and will contribute to continual improvement. So, whilst demonstrating staff have passed their training assessments is a good first step to meet the regulations, it is clearly not reaching the standards that inspections will be looking for – how is that learning carried over into the technical role?
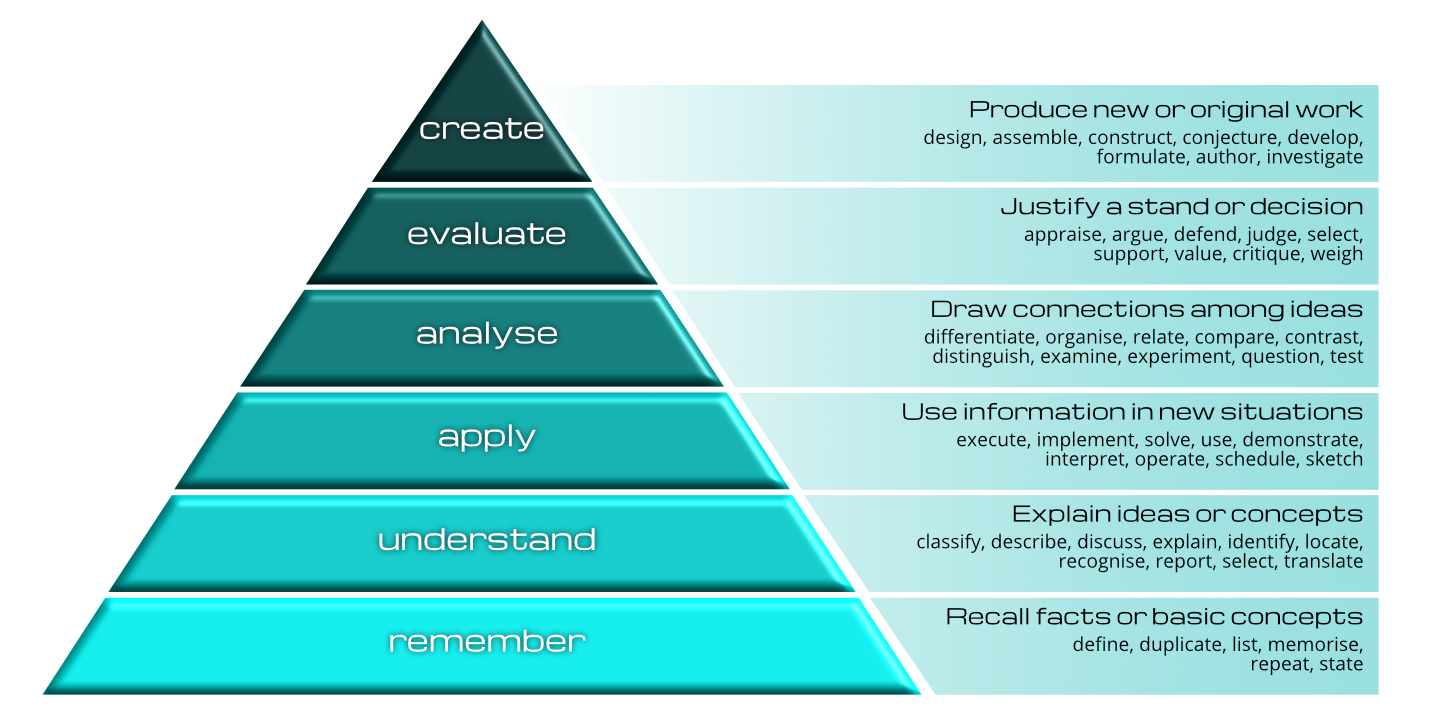
The figure above [1] (courtesy of Vanderbilt University Center for Teaching) shows a pyramid of learning excellence. Progressing through the key stages – remember focuses on fact and memory. A Technical example may be the learning of a process described in an SOP. If learning stops at this point, then the learner, at best, will be able to repeat key statements. Moving on up the pyramid, understand, apply and analyze move through taking the knowledge and applying it in real-life situations such as pharmaceutical environments. This may include making the connection between good documentation and data integrity and the effective management of a product recall. The top stages of evaluate and create are the Holy Grail of learning; this is where learning goes beyond the task in hand and develops the learner into taking a more responsible and perhaps more profitable role within the organisation. For example, an increased focus on the integrity of the pharmaceutical supply chain and the application of quality risk management with a focus on patient safety.
Learning in the Technical Environment
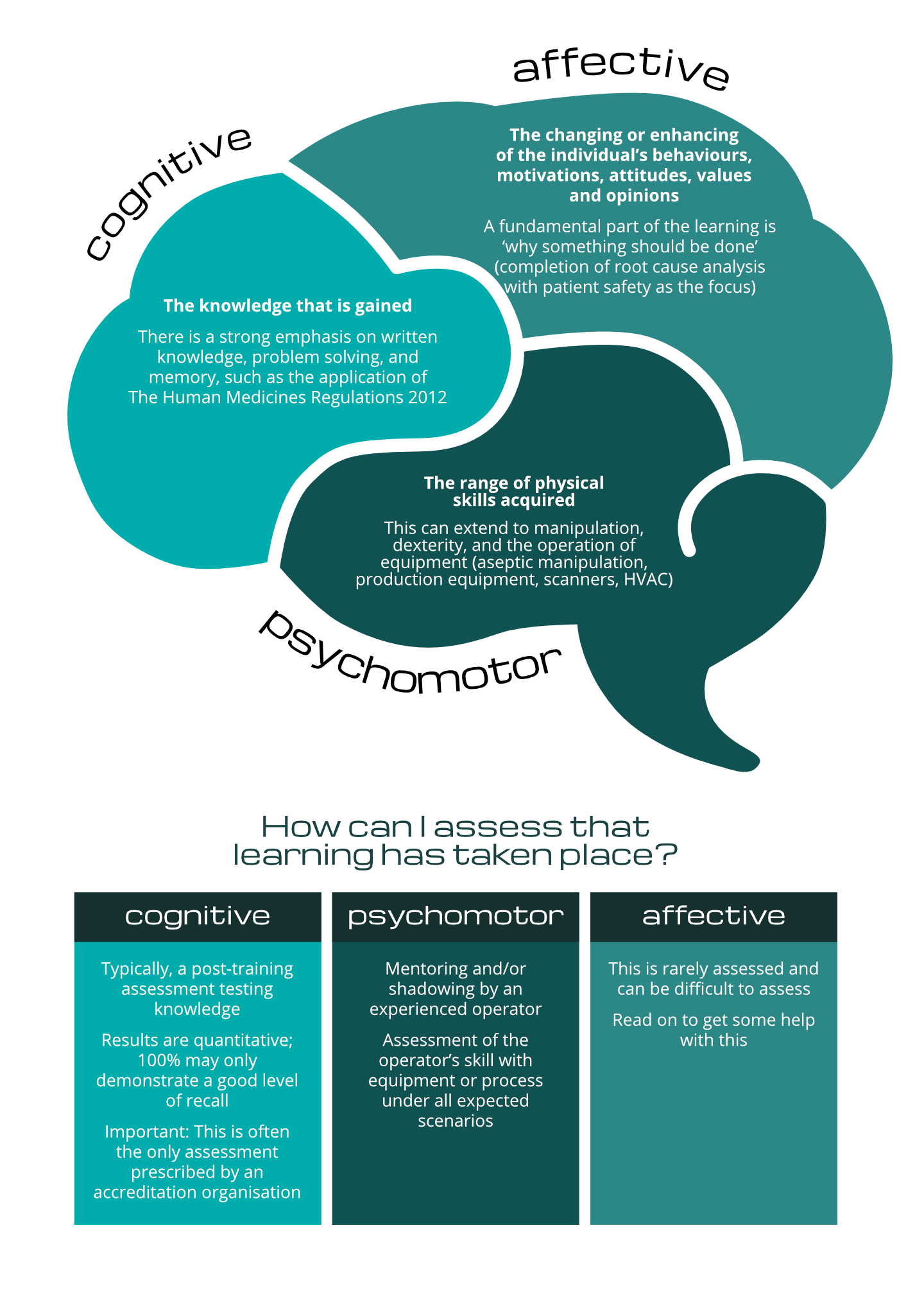
The figure below [2][3] shows three distinct areas of learning to be identified in the technical environment, a description of what that kind of learning is and some common means of assessing this:
The assessment of learning in the ‘affective learning domain’ is so important; but so frequently neglected. This is a massive, missed opportunity.
Experiences of Assessment in the Affective learning Domain in the Technical Environment
Good staff training should ensure all types of learning are covered and assessed. It isn’t enough to supply the knowledge and hope the learner will utilise this in the right way.
In my experience as a teacher in the workplace, I have seen the value of allowing each learner to develop the concepts and learn how to apply them in new situations. I was teaching aseptic dispensing techniques to a group of doctors. It was essential that learning moved beyond the physical drawing up of a liquid into a syringe. It needed to extend to an exploration of the responsibilities of contamination prevention and in developing team skills.
As an MHRA inspector I often saw staff training sessions that were not engaging; they didn’t develop beyond the SOP contents. Staff did not know why something was so important. This is a valuable opportunity missed. These staff will confront situations not described in the Quality System.
At the time I completed my PGCE dissertation in this subject area there was little academic coverage and little experience in the workplace. More recently there has been a greater focus on this. See also Shephard [4], and Boyd [5] which include useful references.
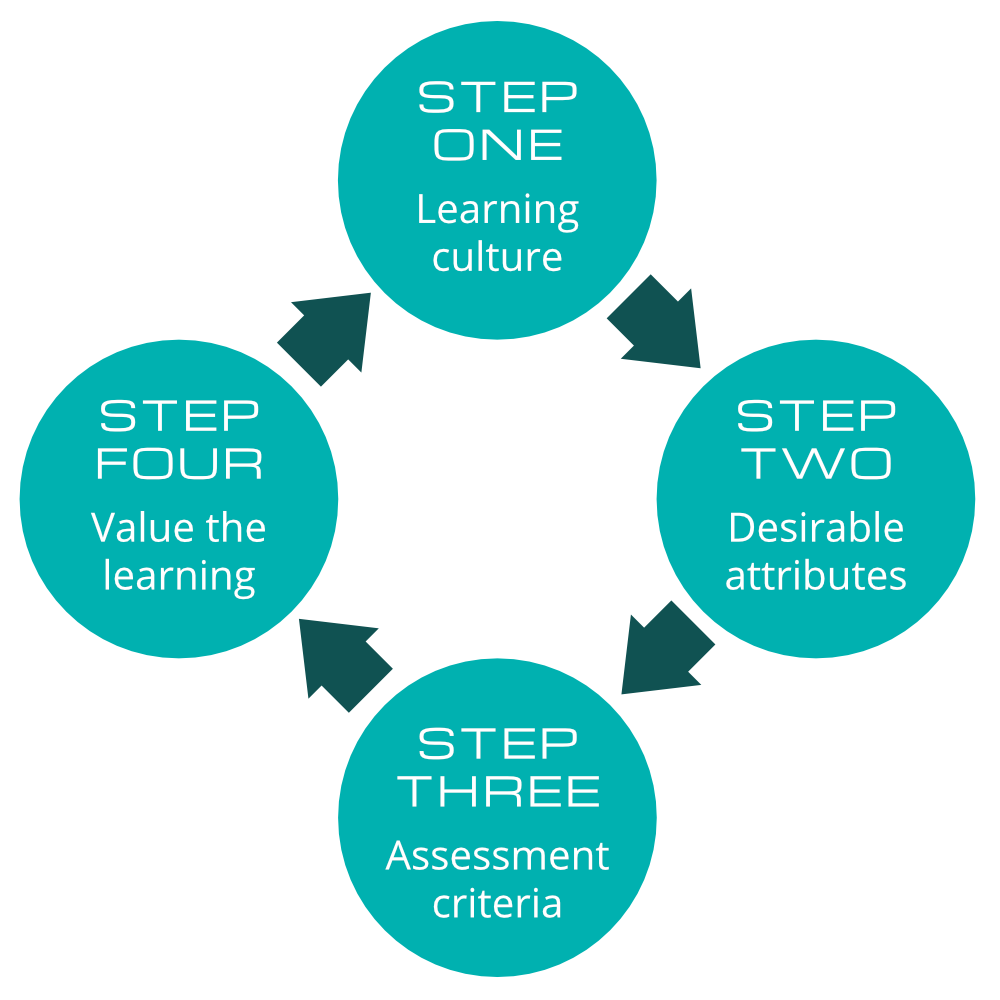
Assessing learning in the affective learning domain – a four-step approach
Being sure that you deliver the three areas of learning and then assess all those areas will give you the right calibre of staff. This assessment must be practical and can be applied by a busy supervisor. This learning should translate to an increased willingness to do it right every time, even in unplanned for circumstances. The learner ’s engagement with quality improvement will be noticeable. The true learning stars are those that develop a passion for the subject and want to share their learning with others; they will want to innovate. A good employer will use this in succession planning – who is the next star that will help the company to innovate? Try this stepped approach:
The four steps of assessing learning in the affective learning domain
Step One:
A good starting point is to think about the Culture of Learning within your organisation. Is there any scope for improvement here? Key attributes of a good Culture of Learning may include:
• A mentoring programme
• Training incorporating a range of providers and resources
• Staff learning led by enthusiastic Subject Matter Experts
• Company recognition of achievements
• A well-developed Succession Planning model
Step Two:
Next you will want to look at your star learners in your own pharmaceutical technical environment. You may notice the following attributes; and perhaps think of more:
• Asking questions
• A personal aim for right every time
• Contribution to a collective quality culture
• An individual who champions the cause
• Seeking out best practice and opportunities to apply
• Structured approach to applying improvements. Not one who avoids the proper channels
• Openness when making a mistake
• Active involvement with managing deviations and CAPA
• Critical questioning of workarounds
• Engaging in challenging discussions
Step Three:
Now develop those attributes into a set of criteria you can use to assess your learners. Make it bespoke to your learners. This assessment would be most effective if performed some time after the training session. Try a combination of interview and observation. Each learner has a different personality and different opportunities so you may not notice all the attributes listed. Be prepared to be flexible and don’t try and make it too quantitative. Having a finger on the pulse of current competence in the workforce is important as it will vary between employees, such that periodic refresher training will be too soon for some, too late for others.
Step Four:
Give praise where it is due; and don’t forget constructive criticism. Show that you value this advanced level of learning. This comes back to the culture of learning.
Conclusion
This article should give you some ideas as to how you can improve the assessment of learning in your pharmaceutical technical environment and the business can benefit not only from tighter compliance standards but a workforce who are more attuned to quality improvement rather than regulatory box-ticking. Developing staff who can justify their decisions and produce new and original work can bolster the organisation’s resilience in the face of changes in the pharmaceutical landscape.
Simple methods, documented in your quality system, can help you see where you are really making a difference with your training. Your staff are your most valuable resource, they will appreciate a focus on their development as a whole.
Tell us at SeerPharma UK what you think?
We would love to hear if you have a method of assessing learning that works particularly well. What do you like about it? What do you want to improve? Have you found this article helpful? Click here for feedback form.
SeerPharma are a provider of training courses, consultancy and audit to the pharmaceutical industry. All work is completed or supervised by former MHRA inspectors. Our experience includes across a wide range of business models, Responsible Person and RPi roles, pharmacy, manufacturing and teaching experience.
Share to Facebook Share to Twitter Share to LinkedIn Share by email
The four steps of assessing learning in the affective learning domain
Step One:
A good starting point is to think about the Culture of Learning within your organisation. Is there any scope for improvement here? Key attributes of a good Culture of Learning may include:
• A mentoring programme
• Training incorporating a range of providers and resources
• Staff learning led by enthusiastic Subject Matter Experts
• Company recognition of achievements
• A well-developed Succession Planning model
Step Two:
Next you will want to look at your star learners in your own pharmaceutical technical environment. You may notice the following attributes; and perhaps think of more:
• Asking questions
• A personal aim for right every time
• Contribution to a collective quality culture
• An individual who champions the cause
• Seeking out best practice and opportunities to apply
• Structured approach to applying improvements. Not one who avoids the proper channels
• Openness when making a mistake
• Active involvement with managing deviations and CAPA
• Critical questioning of workarounds
• Engaging in challenging discussions
Step Three:
Now develop those attributes into a set of criteria you can use to assess your learners. Make it bespoke to your learners. This assessment would be most effective if performed some time after the training session. Try a combination of interview and observation. Each learner has a different personality and different opportunities so you may not notice all the attributes listed. Be prepared to be flexible and don’t try and make it too quantitative. Having a finger on the pulse of current competence in the workforce is important as it will vary between employees, such that periodic refresher training will be too soon for some, too late for others.
Step Four:
Give praise where it is due; and don’t forget constructive criticism. Show that you value this advanced level of learning. This comes back to the culture of learning.
Conclusion
This article should give you some ideas as to how you can improve the assessment of learning in your pharmaceutical technical environment and the business can benefit not only from tighter compliance standards but a workforce who are more attuned to quality improvement rather than regulatory box-ticking. Developing staff who can justify their decisions and produce new and original work can bolster the organisation’s resilience in the face of changes in the pharmaceutical landscape.
Simple methods, documented in your quality system, can help you see where you are really making a difference with your training. Your staff are your most valuable resource, they will appreciate a focus on their development as a whole.
Tell us at SeerPharma UK what you think?
We would love to hear if you have a method of assessing learning that works particularly well. What do you like about it? What do you want to improve? Have you found this article helpful? Click here for feedback form.
SeerPharma are a provider of training courses, consultancy and audit to the pharmaceutical industry. All work is completed or supervised by former MHRA inspectors. Our experience includes across a wide range of business models, Responsible Person and RPi roles, pharmacy, manufacturing and teaching experience.
References and Bibliography
[1] Vanderbilt University Center for Teaching, Bloom’s Taxonomy, https://cft.vanderbilt.edu/guides-sub-pages/blooms-taxonomy, accessed 04 May 2021
[2] Bloom, B.S. and Krathwohl, D.R. (1956), Taxonomy of Educational Objectives: The Classification of Educational Goals, David McKay, New York, NY.
[3] Krathwohl, D.R., Bloom, B.S. and Bertram, B.M. (1973), Taxonomy of Educational Objectives, the Classification of Educational Goals, Handbook II: Affective Domain, David McKay, New York, NY.
[4] Shephard, Kerry, (2007), Higher education for sustainability: seeking affective learning outcomes, Higher Education Development Centre, University of Otago, Dunedin, New Zealand. Shephard accessed 04 May 2021
[5] Boyd, Barry L., Associate Professor, Dooley, Kim E., Associate Professor, Felton, Summer, Assistant Lecturer (2006). Measuring learning in the affective domain using reflective writing about a virtual international agriculture experience, Texas A&M University. Boyd accessed 04 May 2021
Sign up for our regular communications
It’s all here, from must-read articles, savings on training to being the first to know about new courses… of course you need to know!