WHY CAN'T I USE
EX-WORKSWHY CAN'T I USE EX-WORKS
Is there a problem with ex-works for export of pharmaceuticals?
July 2021




Madeleine Ault
Director and Senior Consultant
This is a question I am frequently asked as a consultant so it seemed a good idea to share some information with you all.
Why do the MHRA inspectors say we cannot use ex-works for export of pharmaceuticals? To start with there is nothing in GDP or the Human Medicines Regulations 2012 that says you cannot use ex-works. It doesn’t specifically say why you cannot in the Green Guide (Rules and Guidance for Pharmaceuticals Distributors 2022). So what’s the problem? Is there a problem? Surely this is perfectly legitimate export practice? I shall explain why the MHRA position has been adopted so you can understand how to reduce risks maintain compliance and avoid those unexpected inspection deficiencies.
What is ex-works?
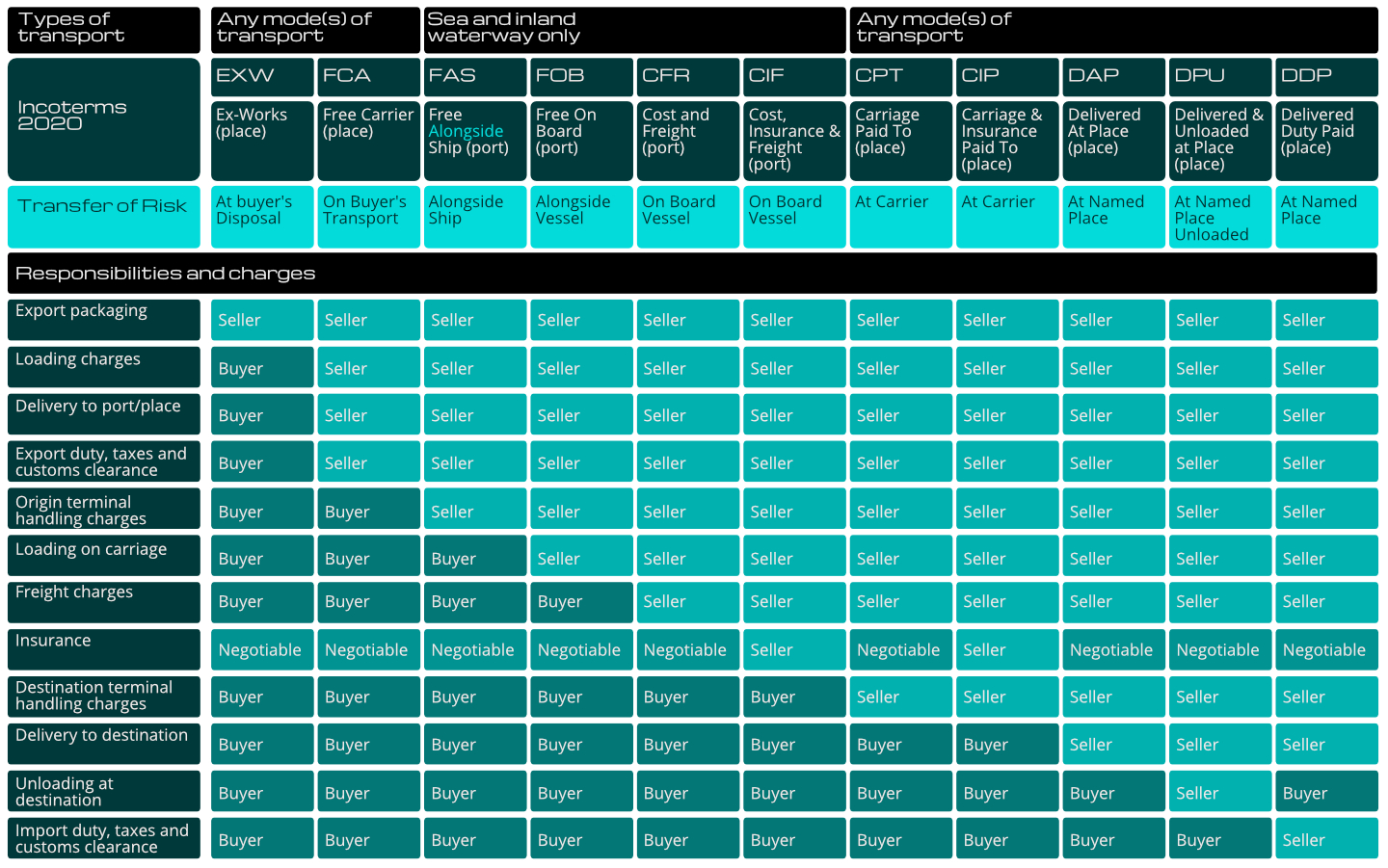
Let’s start with an explanation of what ex-works is. Ex-works (EXW) is one of the Incoterms® 2020, (International Commercial Terms). These are used worldwide to define responsibility and liability for goods imported and exported. The full set of Incoterms® are described in the table below (click to expand).
From this example you can see that each Incoterm® shows the transfer of risk between buyer and seller, their respective responsibilities for packaging, loading, export duty, customs clearance, and in some cases insurance.
Incompatibility with GDP and the Human Medicines Regulations
Often the first time a company becomes aware of this problem is when an inspector visits. The MHRA inspector may raise deficiencies similar to these (I have emphasised the phrases that I will return to):
‘The licence holder was not able to demonstrate that appropriate measures had been taken to ensure exported medicinal product did not reach the UK or EU market. This was evidenced by the customs clearance documentation being completed by another party and in some cases being unavailable for inspection.’ (GDP 4.2 and 5.9)
‘The licence holder distributed medicinal products, by way of wholesale dealing, to a person not authorised to receive the product in the UK. This was evidenced by an ex-works transaction to XYZ’. (GDP 5.3 and Human Medicines Regulations 2012, Regulation 44 (5))
‘The licence holder did not put in place measures to ensure protection of medicinal products against breakage, adulteration, theft, and extremes of temperature. This was evidenced by there being no oversight of the arrangements for transport of medicines to XYZ’. (GDP 9.1)
Let’s take a closer look at the ex-works choice that has led to these deficiencies: Considering EXW in the Incoterms® diagram, we can see that the seller choosing the EXW option ensures the buyer is responsible for everything except the export packaging. As a seller, this might seem a good thing – you sell the product, and it is off your hands! This is where you will find incompatibility with GDP and the Human Medicines Regulations 2012 in the following three respects:
1 Customs clearance
If the supply is made as an ex-works transaction, then the purchaser is taking responsibility and risk from the warehouse door of the seller’s premises. One of these responsibilities is for customs clearance. Whether you do this yourself or outsource it to your freight agent who performs it under your oversight, you, as a seller of a pharmaceutical are the exporter and must take responsibility for customs clearance. By allowing another person to take responsibility for customs clearance, you are not able to provide evidence that the medicines have left the UK and so you are not complying with ‘Wholesalers should take the appropriate measures in order to prevent these medicinal products reaching the Union market’ (GDP 5.9). Even though the UK has now left the EU, it would be expected that this is interpreted as preventing medicinal products from returning to the UK. It also important to note that the EXW option places no obligation on the buyer to supply the customs clearance evidence to the seller.
2 Persons not authorised
By making a supply at your warehouse door you are supplying a person in the UK. This is, therefore, not an export transaction at all. The persons you supply must be ‘persons who are themselves in possession of a wholesale distribution authorisation or who are authorised or entitled to supply medicinal products to the market’ (GDP 5.3 and similar wording in HMR 2012 Reg 44 (5) (a-d)). If there is no UK authorisation or entitlement to receive those medicines, then it is a clear contravention of GDP and the Human Medicines Regulations 2012.
3 Protection of medicinal products
A UK wholesaler is required ‘to protect medicinal products against breakage, adulteration and theft, and to ensure that temperature conditions are maintained within acceptable limits during transport’ (GDP 9.1). It can be acceptable for the buyer to arrange and pay for suitable transport that provides this level of protection, but the seller must have appropriate oversight of this. This should include an assessment of the transport provider to confirm suitable assurance that products are handled in compliance with GDP during transport. That oversight should include evidence of temperature conditions in transit, evidence and access to the associated export documentation, and evidence that product has arrived safely. The responsibility for this, as required in GDP, remains with the seller.
MHRA blog
Read more in a blog from Cheryl Blake, Lead Senior GDP Inspector at the MHRA about the risks of false, incomplete HMRC declarations; the documentation to supply your freight agent; and the documentary evidence required on inspection:
MHRA Blog: Export and Customs Procedures
What do other countries in Europe do?
There are reports that different countries within the EU/EEA adopt differing approaches for the use of ex-works. It is sometimes the case that countries transposing EU Directives into National law will vary in their interpretation. This is difficult for the larger company groups because the Licence Holder/Responsible Person/key staff may be under pressure to use this EXW Incoterm®. The best approach here is for the UK Responsible Person to share this article with the decision makers in your company group so they can fully understand why this is not accepted in the UK. This explanation, together with the risk of unwanted inspection deficiencies, should be persuasive. In addition, there is a risk to the integrity of the wider supply chain, so promoting public health is our next consideration.
Supplies from Northern Ireland to the EU
Since Brexit supplies to the EU are not so easy. The UK is considered a third country by the receiving country and so the importer in the EU country is likely to need to have an MIA to import medicines from outside the EU. This may prevent the UK WDA holder from making that transaction. Where supplies are made from Northern Ireland to the EU whatever type of licence is held by the supplier or customer the supplier should carefully consider all three incompatibility aspects above to determine how the supplier can continue to be compliant. Any doubt should be discussed with your last inspector or raised with gdp.inspectorate@mhra.gov.uk
A risk to the integrity of the supply chain
It may seem as if you are being made to jump through additional hoops for no good reason but there is a genuine cause for concern. It is not uncommon for distributors in the UK to be approached by third parties claiming to be in a third country. They want to buy medicinal product from you, and they prefer to send a courier to collect it and complete the customs declaration themselves. This should ring alarm bells as it is most likely they are not the person in the third country that they claim to be, and they want to buy product for distribution on the UK black market – the medicines, often controlled drugs, will never be exported and are diverted into the illegal supply chain and may contribute to substance abuse in the UK.
As many of the medicinal products in question are controlled drugs, the risk of non-compliance with Home Office Controlled Drug requirements is a further consideration not to be ignored.
Article updated 23.12.2022
Learn from the people who know
This article should have helped you to understand the implications of your export department choosing EXW, a commonly recognised Incoterm®. An export department making a poor choice may be the trigger for additional inspector scrutiny and may raise questions over the control the Licence Holder and Responsible Person have over the integrity of their supply chain. This article describes the current MHRA position at the time of writing, readers should be aware that this position may change in the future.
SeerPharma offer a range of training courses as well as bespoke training. Visit our training page for more details or contact us directly for more information about how we can help.
Sign up for our regular communications
It’s all here, from must-read articles, savings on training to being the first to know about new courses… of course you need to know!